Energy and
Environmental
Research
Laboratory
Estejab Research Group
Exploring the beauty of science for a sustainable future through catalytical activity
"The important thing is not to stop questioning."
Albert Einstein

Photo by Alan Rodriguez on Unsplash
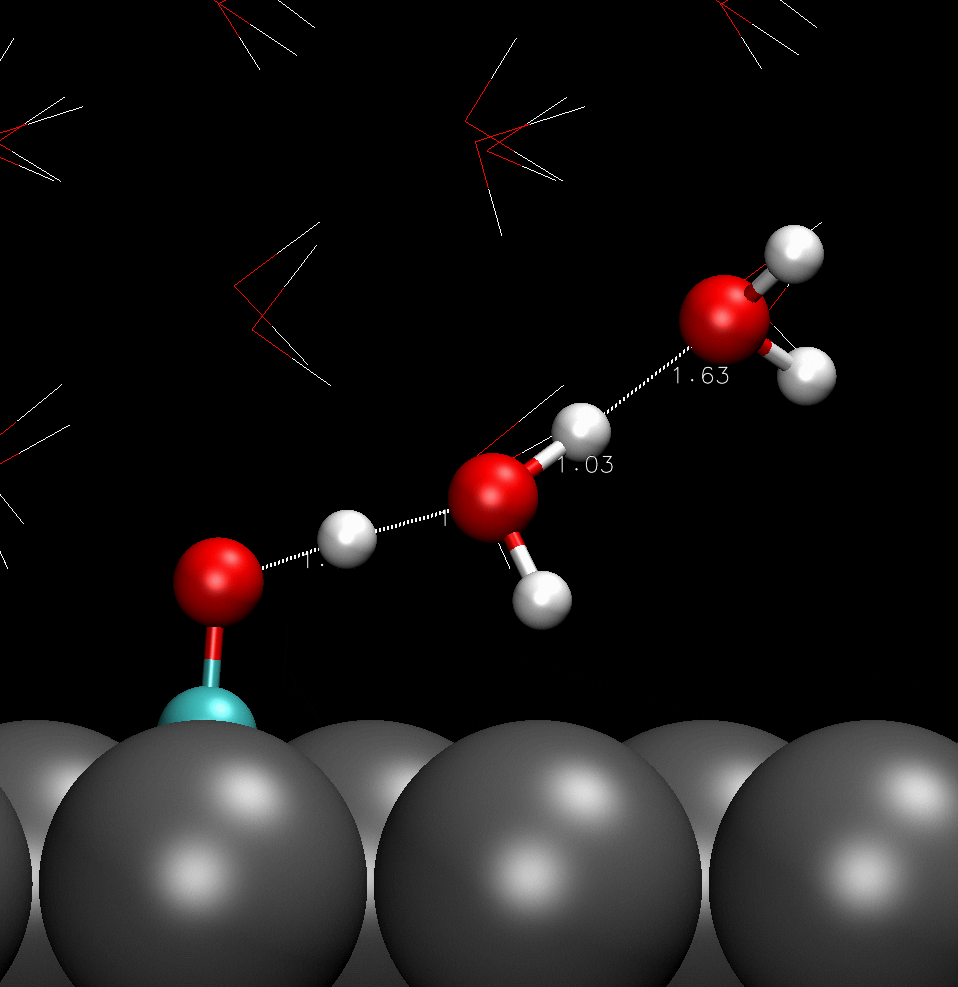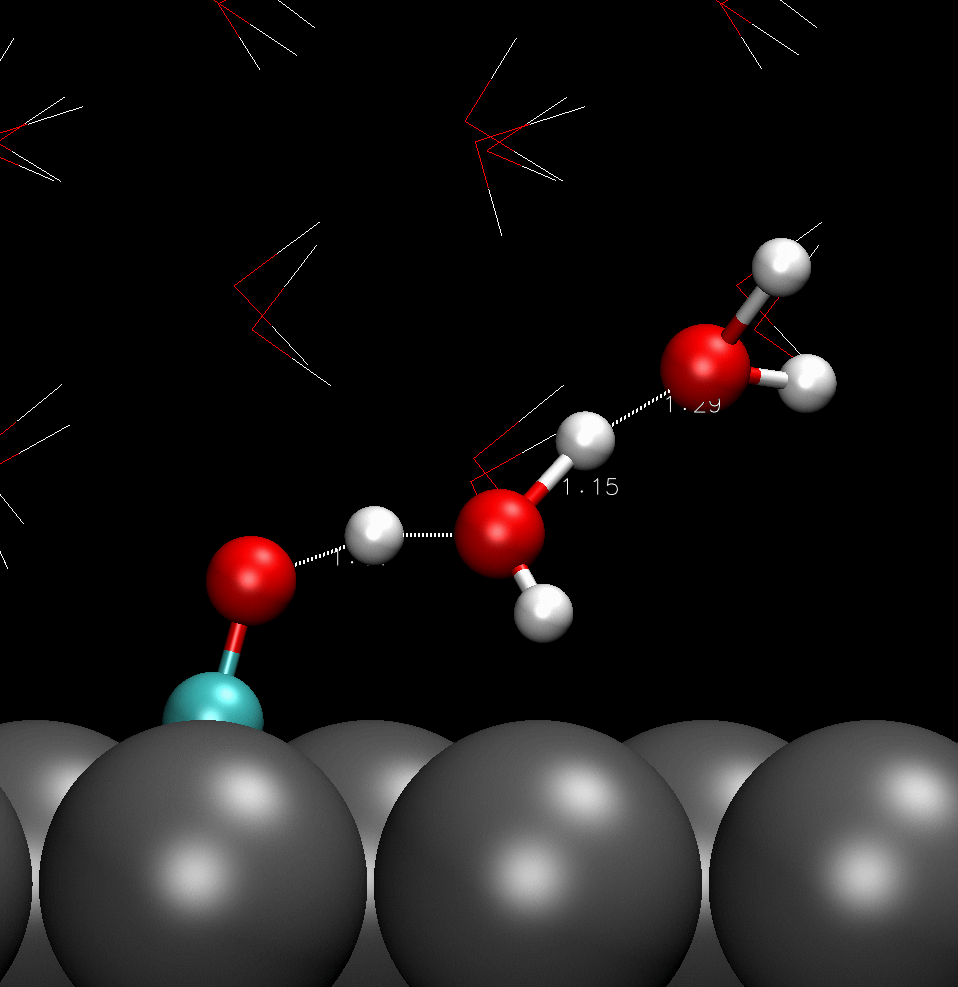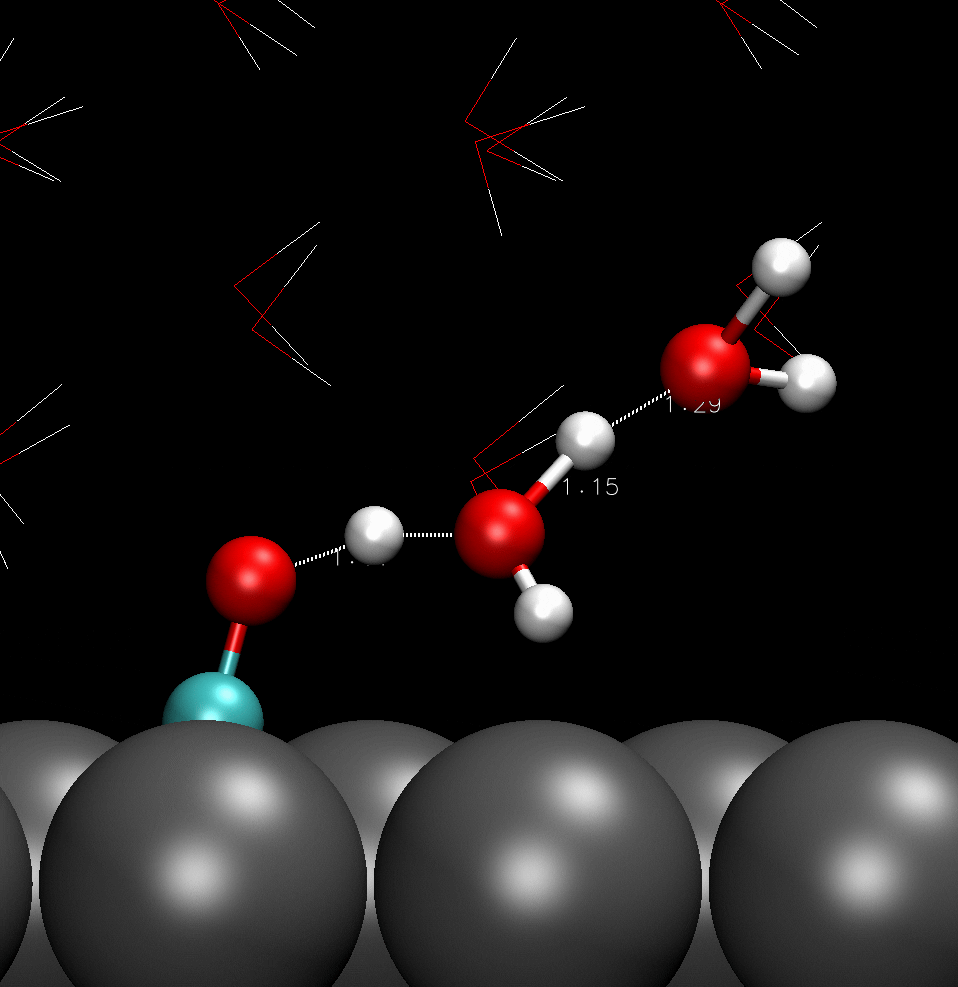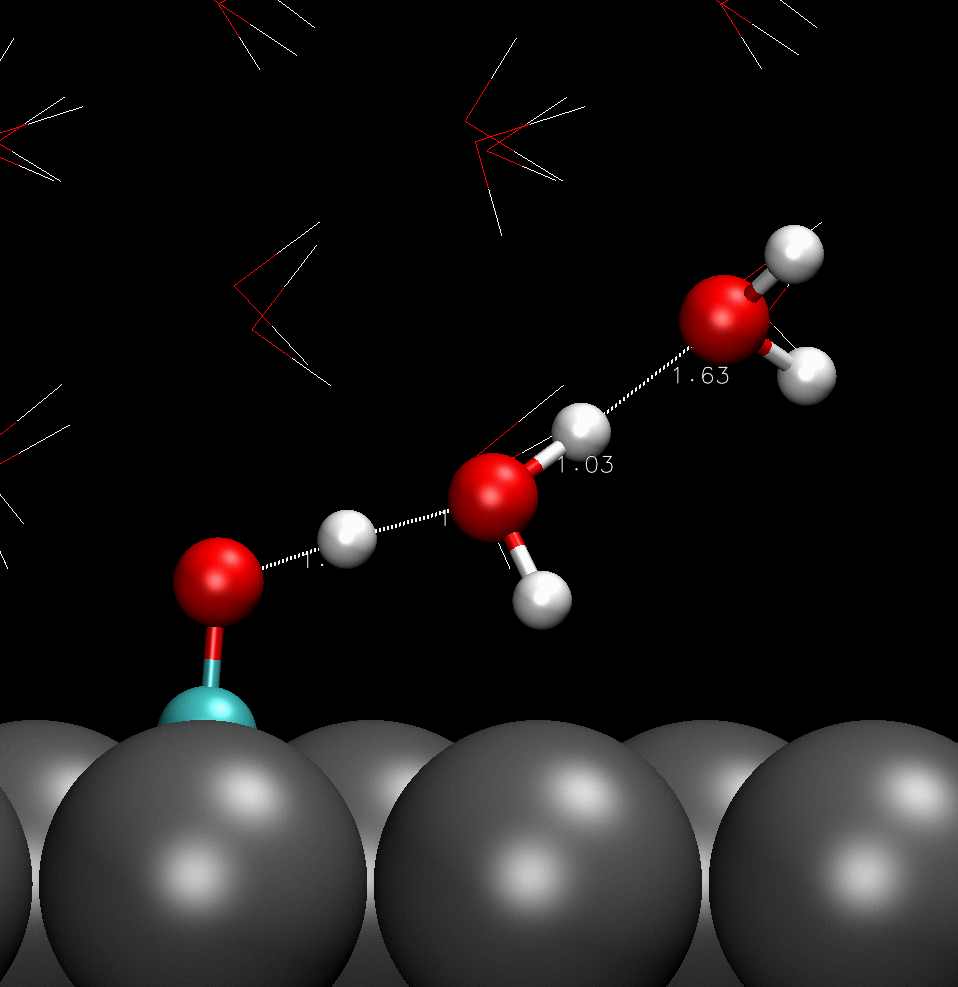
COH adsorbed on catalyst surface; hydrogen boned with adjacent water molecules.
Secrets of Science
To address environmental challenges like pollution from fossil fuel use, we focus on catalysis and novel materials for pollutant separation. Additionally, we explore green energy and novel methods in added-values of chemical products. Our research spans computational techniques, including macro and micro scales, and atomic and molecular scales using quantum mechanics and molecular dynamics. Combining industrial experience with academic pursuits, we seek practical solutions to complex environmental issues.

https://news.mit.edu/
Applications of Molecular Modeling
New generation of catalyst and separation techniques
Metal-Organic Frameworks (MOFs) have emerged as a promising and innovative approach for the separation of pollutants; although, their application does not limit to separation. They can be used as catalysis, gas storage and drug delivery.
They are suitable for gas storage and separation via adsorption due to their high surface area and large pore volume.
Other Applications of Molecular Modeling

thecentraltrend.com
What is going on the Mars?
When planning for missions to Mars, one critical challenge is protecting spacecraft and astronauts from the harsh Martian environment, which includes high levels of radiation and extreme temperature fluctuations. To address this, chemical engineers use molecular modeling to design and optimize advanced materials for insulation and radiation shielding.
By virtually studying the interactions between different materials at the molecular level, researchers can identify compounds that exhibit exceptional thermal resistance, low thermal conductivity, and high radiation absorption properties.
They can also explore the behavior of these materials under Martian conditions to ensure their durability and performance during the long and challenging journey to and from Mars.
Photo by National Cancer Institute on unsplash
Designing new drugs using molecular modeling.
By virtually exploring how the drug molecules interact with specific targets in the body, we can predict their effectiveness and side effects, streamlining the drug development process.
Projects
Conversion of harmful pollutants into valuable products
Using molecular modeling techniques, you can simulate the interactions between the catalyst's surface and the reacting molecules. i.e. as the world's population grows, the demand for agricultural products increases, necessitating more urea production for fertilizers. To achieve this, we require NH3 (ammonia) and CO2 (carbon dioxide) as key inputs. NH3 can be produced through various methods, including the Fischer-Tropsch process, albeit with high temperatures and pressures.
Alternatively, NH3 can be generated by utilizing NOX (nitrogen oxides) and CO (carbon monoxide), which not only aids in urea production but also removes contaminants from the environment.
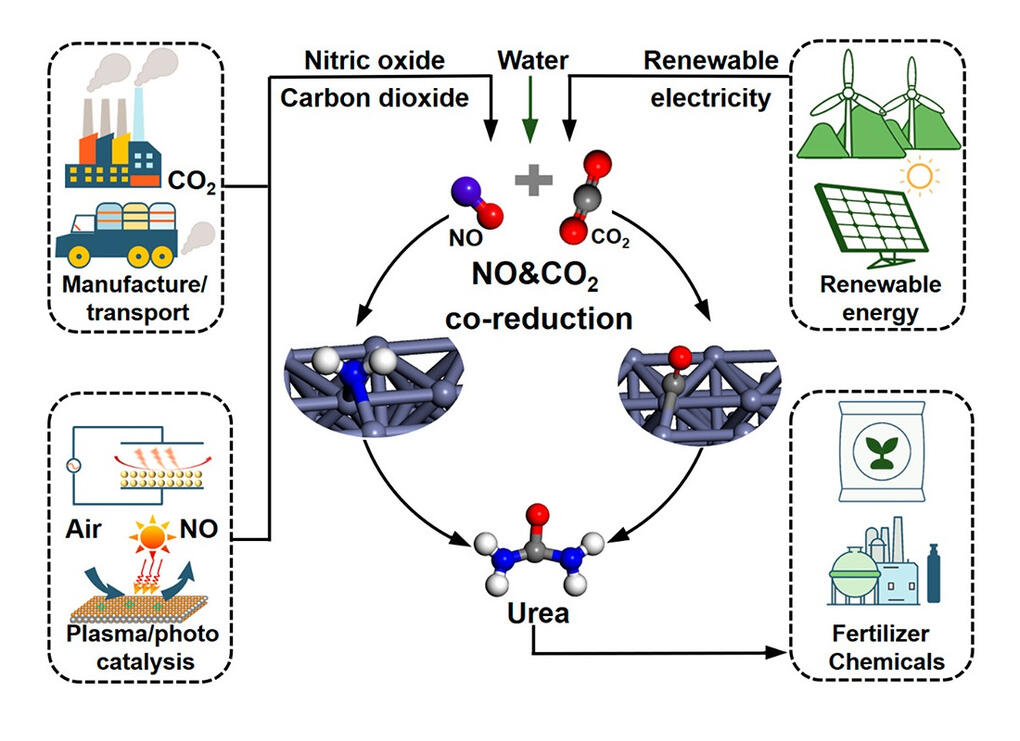
ACS Energy Lett. 2022, 7, 1
By combining NH3 and CO2, we produce urea, a valuable nitrogen-based fertilizer. Utilizing NOX and CO for urea production helps achieve both environmental benefits and increased agricultural output to meet the growing global demand.
Crystallization and Molecular Modeling
The shortage of freshwater for consumption and irrigation is a pressing issue in many regions globally, including parts of the U.S. Simultaneously, efforts to reduce carbon emissions through clean energy and electric vehicle production have heightened the demand for energy storage, leading to a shortage of lithium for lithium-ion batteries.
The demand for lithium and its compounds, has surged in recent years. High-purity lithium salts is essential for manufacturing battery materials like cathodes and electrolyte. It can be sourced from lithium-containing minerals and brines. However, significant amounts of unextracted lithium are discarded from these processes, leading to environmental concerns and resource wastage. Similarly, desalination processes generate liquors with valuable minerals that are often discarded.

Photo by Jason D on Unsplash
The crystallization is an important and critical process for recovering valuable compounds from various solutions. Efficient crystallization methods are essential for industries where precise control of crystal size, purity, and yield is required. We utilize Molecular dynamics (MD) and quantum mechanics (QM) simulations to explore the crystallization process. The main objective is to investigate how factors like the orientation of the molecules, the system's temperature, and the solution's composition will impact crystal growth. Specifically, we analyze whether the presence of additional compounds will influence the properties of the resulting crystals.
Opportunities
Research OpportunitiesWe are currently looking for highly motivated Ph.D. and M.S. students who are passionate about research and eager to push the boundaries of knowledge in catalysis and sustainable chemistry. Ideal candidates should be committed to scientific rigor, possess strong computational and analytical skills, and have a genuine interest in tackling pressing environmental challenges. If you are interested in joining a dynamic research group where collaboration, innovation, and growth are highly valued, please reach out through "Contact" section — We look forward to exploring new possibilities together.

Photo by Eric Prouzet on Unsplash
Publication and Presentation
Publications (most recent)R, A. Garcia Carcamo, J. Shi, A. Estejab, T. Xie, S. Bhattacharjee, S. Biswas, C. J. Bodenschatz, X. Chen, M. Maurya, X. Zhang, R. B. Getman, A Perspective on Multiscale Modeling of Explicit Solvation-Enabled Simulations of Catalysis at Liquid–Solid Interfaces, ACS Catalysis, V. 15, p. 7448-7457, April 2025Ali Estejab, Rahel B. Getman, Water/Solid Interface in Thermal-and Electrocatalysis for Wetting and Non-Wetting Surfaces: Interactions and Models. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier, 2023.Ricardo A Garcia Carcamo, Xiaohong Zhang, Ali Estejab, Jiarun Zhou, Bryan J Hare, Carsten Sievers, Sapna Sarupria, Rachel B Getman, Differences in Solvation Thermodynamics of Oxygenates at Pt/Al2O3 Perimeter versus Pt(111) Terrace Sites. iScience 2023, 26 (2), 105980.Ali Estejab, Ricardo. A. García Cárcamo, and Rachel B. Getman, Influence of an electrified interface on the entropy and energy of solvation of methanol oxidation intermediates on platinum(111) under explicit solvation, Physical Chemistry Chemical Physics, 2022, 24, 4251 – 4261.Ali Estejab, and Gerardine G. Botte, Ammonia oxidation kinetics on bimetallic clusters of platinum and iridium: a theoretical approach, J. of Molecular Catalysys A: Chemical, 445, pp. 279-292, 2018.Ali Estejab, and Gerardine G. Botte, DFT calculations of ammonia oxidation reactions on bimetallic clusters of platinum and iridium, J. of Computational and Theoretical Chemistry. 1091, p31, 2016.Ali Estejab, Damilola A. Daramola, and Gerardine G. Botte, Mathematical model of a parallel plate ammonia electrolyzer for combined wastewater remediation and hydrogen production, Water Research, vol. 77, pp. 133-145, 2015.
.
.
Presentations (most recent)Ali Estejab, “Effects of Electrified Interface and Solvation on Ammonia Dissociation Reaction”, AIChE Boston, 2025.Ali Estejab, Ricardo A. García Cárcamo, Rachel B. Getman, “Electrified Interface influence on the Entropy and Energy of Solvation of Methanol Oxidation Intermediates on Platinum(111) Under Explicit Solvation.” NAM27 New York, May 2022.Ali Estejab, Rachel B. Getman, “Influence of an external potential on the solvation thermodynamics of intermediates in the pathway for methanol oxidation on Pt(111)”, AIChE Boston, November 2021.Ali Estejab, Rachel B. Getman, “Effect of potential and explicit aqueous media on oxidation process on Pt(111) using computational multi-scale modeling”, SECS virtual symposium September 2020 and AIChE virtual meeting November 2020.Ali Estejab, Gerardine G. Botte, “Theoretical calculations of ammonia oxidation kinetics on platinum, iridium and their bimetallic clusters. ECS Conference, San Diego, May-June. 2016Ali Estejab, Gerardine G. Botte, “Optimized performance of a scale-up ammonia electrolyzer”, ECS Conference, Chicago, May. 2015
.
.
.
.
.
.
.
.
.
.
.